|
How do you get francium?
Naturally occuring francium is difficult to study
since it must be quickly and efficiently extracted from the sample in which it
is embedded. In practice, francium studies require its laboratory production.
At Stony Brook, we produce francium in a heavy ion nuclear fusion reaction at
the Stony Brook Nuclear Structure Laboratory's superconducting linear
accelerator (LINAC).
We produce francium by colliding or fusing 100 MeV oxygen nuclei (18O)
with gold nuclei (197Au) in a stationary target:
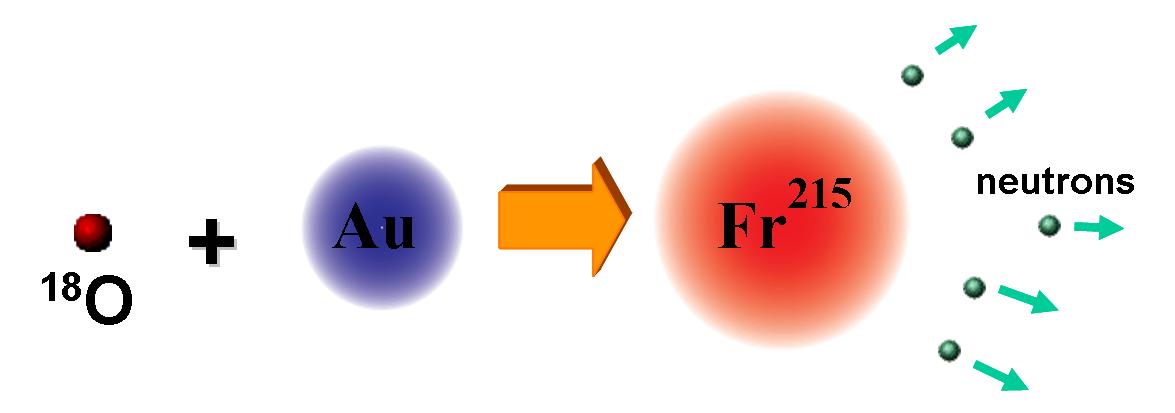
Fusion reaction for producing francium.
The fusion reaction produces 215Fr with a lot of
internal energy, which is then released as neutrons. The number neutrons boiled
off can be changed by tuning the energy of the incident 18O
ions. Using this reaction, we can produce 208Fr,
209Fr, 210Fr, and 211Fr.
By substituting gold for platinum in the target, we can produce 212Fr.
In practice, we achieve the highest production rates with 210Fr
(with a half-life of 3 minutes).
The neutrons produced during the fusion reaction
pose a significant health hazard. In order to work in a neutron-free
environment, we remove the francium from the target as an ion and transport it
to a trapping room, located 10 m away behind a 1 m thick concrete wall. When
the francium atoms diffuse out of the target, the gold surface work function
strips the francium of its valence electron. The francium ion is then
accelerated accross a 5 KeV potential and guided electrostatically to the
trapping room:
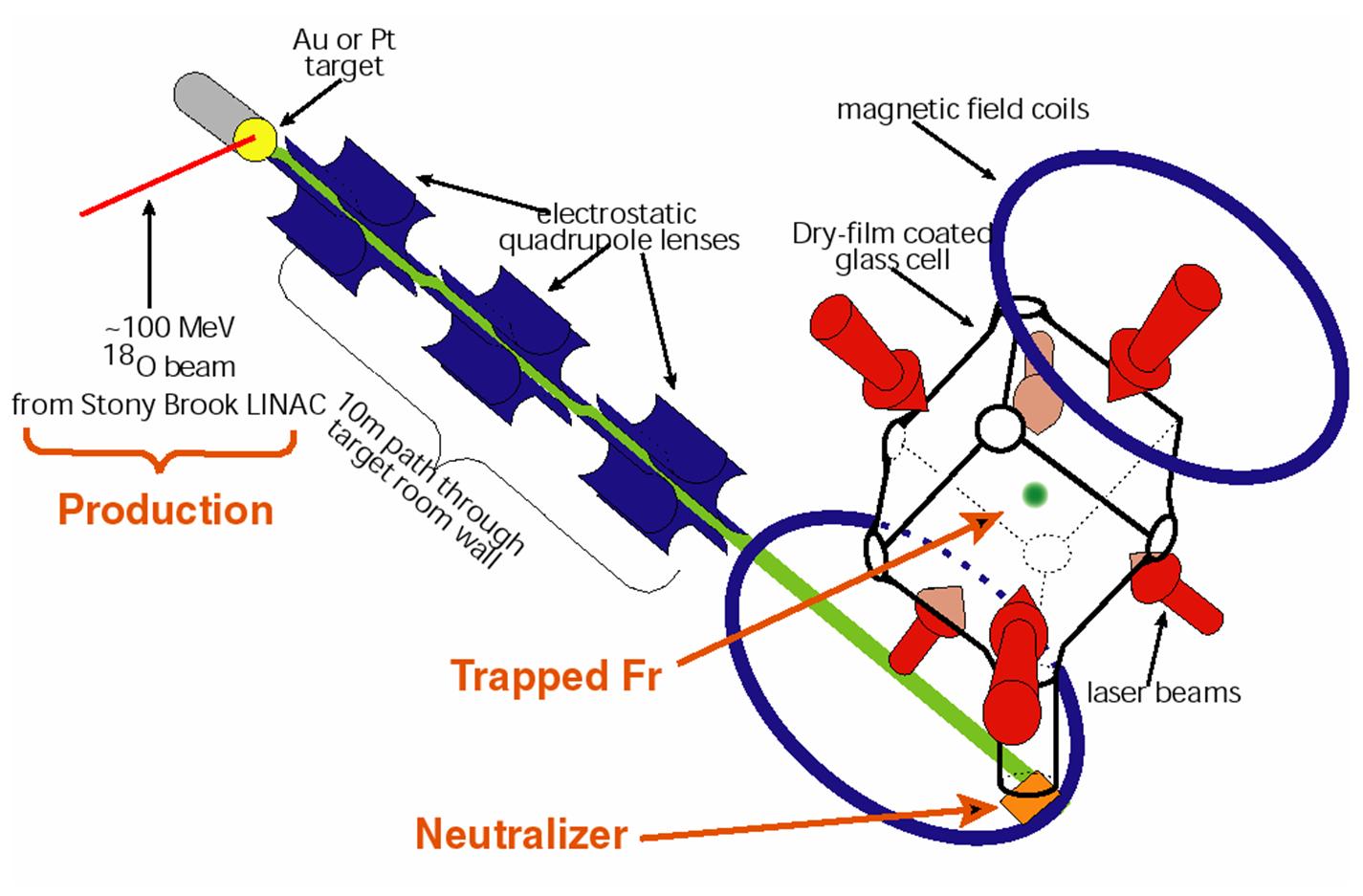
Francium production and trapping apparatus.
The electrostatic optics of our transport beamline ensure a mass independent
transport of all francium isotopes and even other alkalis, such as rubidium,
which we use for testing most of our apparatus.
|